-
The information on this page is current as of April 1 2020.
21 Cfr Part 820 Pdf
820.5 Quality system. § 820.5 Quality system. Each manufacturer shall establish and maintain a quality system that is appropriate for the specific medical device(s) designed or manufactured, and that meets the requirements of this part.
Seiko clock model no qhr016 manual. For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR).
21 Cfr Part 820 Preamble Pdf


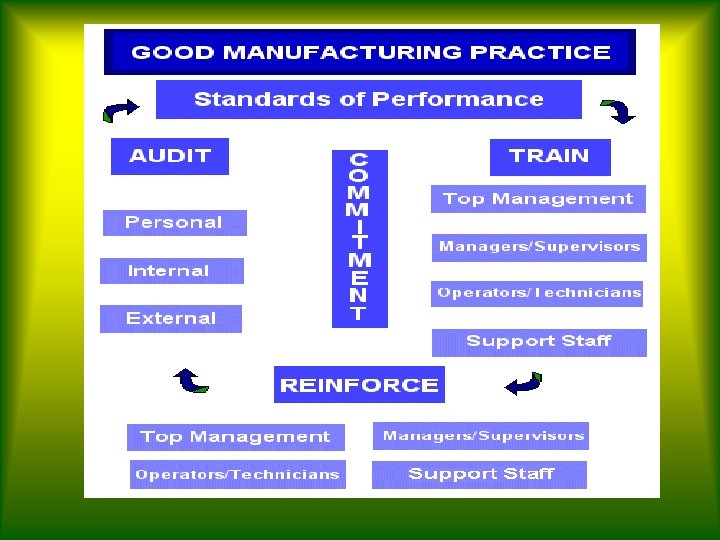
- CFR - Code of Federal Regulations Title 21. Education Details: Apr 01, 2020 For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). § 820.1 - Scope. § 820.3 - Definitions. § 820.5 - Quality system. § 820.20 - Management responsibility. § 820.22 - Quality audit. § 820.25 - Personnel. § 820.30 - Design controls. § 820.40 - Document.
- Electronic Code of Federal Regulations (e-CFR) Title 21 - Food and Drugs; CHAPTER I - FOOD AND DRUG ADMINISTRATION, DEPARTMENT OF HEALTH AND HUMAN SERVICES; SUBCHAPTER H - MEDICAL DEVICES; PART 820 - QUALITY SYSTEM REGULATION; Subpart M - Records § 820.198 Complaint files.
- CFR - Code of Federal Regulations Title 21. Education Details: Apr 01, 2020 For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). (a) Applicability. (1) Current good manufacturing practice (CGMP) requirements are set forth in this quality system regulation.
- Subpart H - Acceptance Activities (§§ 820.80 - 820.86) Subpart I - Nonconforming Product (§ 820.90) Subpart J - Corrective and Preventive Action (§ 820.100) Subpart K - Labeling and Packaging Control (§§ 820.120 - 820.130) Subpart L - Handling, Storage, Distribution, and Installation (§§ 820.140 - 820.170).

|